Abstract
Background
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by auto-antibody induced platelet (PLT) destruction and reduced PLT production, leading to a low PLT count. The availability of robust epidemiological and clinical data on ITP in regions outside Europe and the United States is limited. The International ITP Registry is a prospective cohort study which seeks to collect epidemiological and clinical data of recently diagnosed primary ITP adult patients, predominantly from the Asia-Pacific region. The International Registry has been established for > 6 years.
Aims
To contrast and compare the International ITP Registry population with the Intercontinental Cooperative Immune Thrombocytopaenia Study Group Pediatric and Adult Intercontinental Registry on Chronic ITP (PARC-ITP Registry) adult population. The PARC - ITP Registry was selected as it represents a prospective cohort including newly diagnosed ITP patients, with a comparably sized adult cohort which is well established (>5 years) across the regions in which epidemiological data is already available1.
Method
Clinical and laboratory data from the International ITP Registry 2016 data analysis were compared with clinical and laboratory data reported for adults enrolled in the PARC-ITP registry.
Results
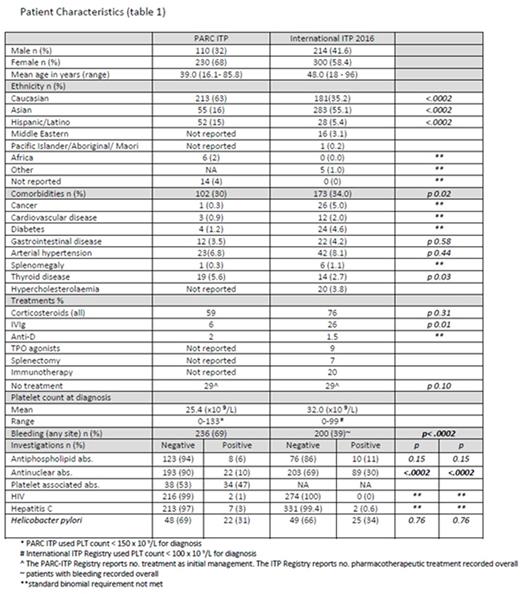
The PARC Registry had 340 adult patients enrolled in 31 countries across Europe, Canada, United States, Latin America, the Middle East, and Asia. As at 31 August 2016 514 patients had data available for the International ITP Registry from 44 sites across 10 countries. Patient characteristics are presented in Table 1.
The International ITP Registry has a predominance of patients of Asian origin whereas the PARC- ITP Registry population is principally Caucasian (55% v 63%). A female predominance is seen in both cohorts.
Comorbidities are reported more often in the International Registry group (34% vs 30% in PARC). Hypertension is the most often reported comorbidity in both groups. Of the comorbidities related to ITP diagnosis that are available for comparison, diabetes occurs more often in the International Registry group and gastrointestinal disease is reported at similar rates in both groups (Table 1).
Mean PLT count at baseline was 32 x 109/L (range 0-99 x 109/L) in the International Registry group and 25.4 x 109/L (range 0-133 x 109/L) in the PARC Registry adults.
Corticosteroids are the most frequently used first-line treatment in both groups - 76% in the International Registry and 59% in the PARC-ITP Registry adults. IVIg is used in both populations at much lower rates - 26 % in the International Registry and 6% in the PARC-ITP Registry adults. Splenectomy is uncommon, with 40 patients in our group having splenectomy, splenectomy is not reported on in the PARC-ITP Registry publication. TPO-receptor agonist use is reported in the International Registry group (n = 48) but not reported on in the PARC-ITP Registry group. Anti-D is reported both groups at rates <5%, and both groups have similar rates of not treated patients with approximately one-third of patients untreated in each group (Table 1).
Clinical signs of bleeding were assessed by location in both Registries. The International Registry also records severity of bleeding. Bleeding at any site was seen in 39% of patients in the International Registry whereas PARC -ITP Registry reports manifestations of bleeding in 69% of adults.
Summary/Conclusion
Comparative data showed similarities in gender distribution, presenting platelet counts, and diagnostic testing, whereas differences occurred in ethnicity, co-morbidity, bleeding, and first and second line therapies.
References
Kühne T, et al on behalf of the Intercontinental Cooperative ITP Study Group. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica 2011;96(12):1831-1837. doi:10.3324/haematol.2011.050799
Lee: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Member of advisory Boards for Novartis on Revolade ;involved in the Novartis sponsored Phase IV multicenter, observational study in Chronic Immune Thrombocytopenia patients on Revolade treatment in emerging markets (CITE Study)", Research Funding. Ilhan: Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal